
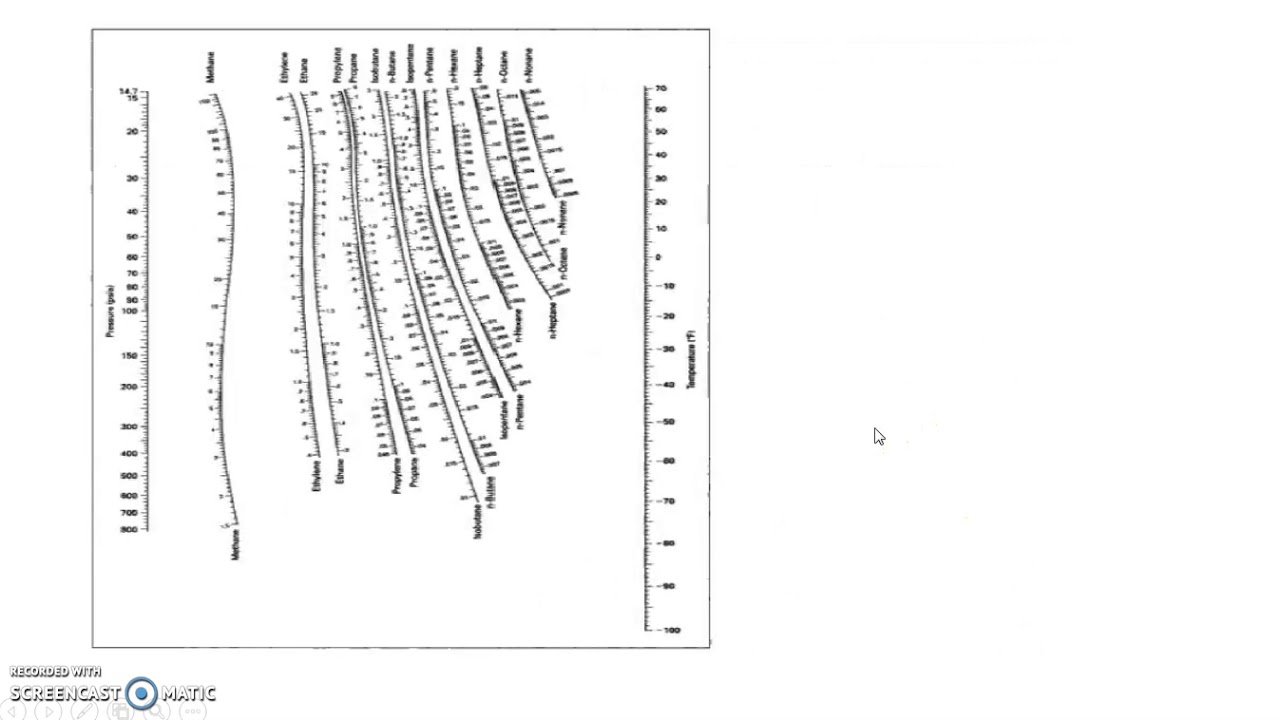
Alternatively, there are several graphical or numerical tools that are used for determination of K-values. Obviously, experimental measurement is the most desirable however, it is expensive and time consuming. For the more volatile components the Kvalues are greater than 1.0, whereas for the less volatile components they are less than 1.0.ĭepending on the system under study, any one of several approaches may be used to determine K-values. Equation (2) is also called 'Henry's law' and K is referred to as Henry's constant. Ki is called the vapor–liquid equilibrium ratio, or simply the K-value, and represents the ratio of the mole fraction in the vapor, yi, to the mole fraction in the liquid, xi. (1) is transformed to a more common expression which is The thermodynamic equilibrium between vapor and liquid phases is expressed in terms equality of fugacity of component i in the vapor phase, f i V, and the fugacity of component i in the liquid phase, f i L, is written asĮquation (1) is the foundation of vapor-liquid equilibrium calculations however, we rarely use it in this form for practical applications. Modeling and design of many types of equipment for separating gas and liquids such as flash separators at the well head, distillation columns and even a pipeline are based on the phases present being in vapor-liquid equilibrium. De Priester Charts Calculator Online Calculator.De Judas Priest Painkiller GfK Entertainment Charts. Australian ARIA Chart peaks: Top 50 peaks: australian - charts com Maxi Priest in Australian Charts 2015. Judas Priest The Official Charts Company Norvegian Chart. Archived from the original on 22 March 2012. Judas Priest Epitaph Tour 2011 Glasgowvant.These nomograms have two vertical coordinates, one for pressure, and another for temperature. DePriester in an article in Chemical Engineering Progress in 1953. DePriester Charts provide an efficient method to find the vapor-liquid equilibrium ratios for different substances at different conditions of pressure and temperature.


 0 kommentar(er)
0 kommentar(er)
